Is Breaking Solvent Solvent Interactions Exothermic
It can be considered as enthalpy of solvation with the solvent being water. Acetone polish remover works by breaking down nail polish and removing it from the nail plate surface.
Solved A Solution Is Formed When The Solute Uniformly Chegg Com
The liquids must have very different polarities.
. Such small energies can be absorbed as vibrations of the lattice dissipated as thermal motion a molecule bouncing across the surface will gradually lose all its energy finally adsorb to it. Hydration enthalpy is also called hydration energy and its values are always negative. Recent development of solvent acid resistant membranes has provided core materials for green sustainable liquid-liquid extraction of Li from the high MLR Chinese salt-lake brine.
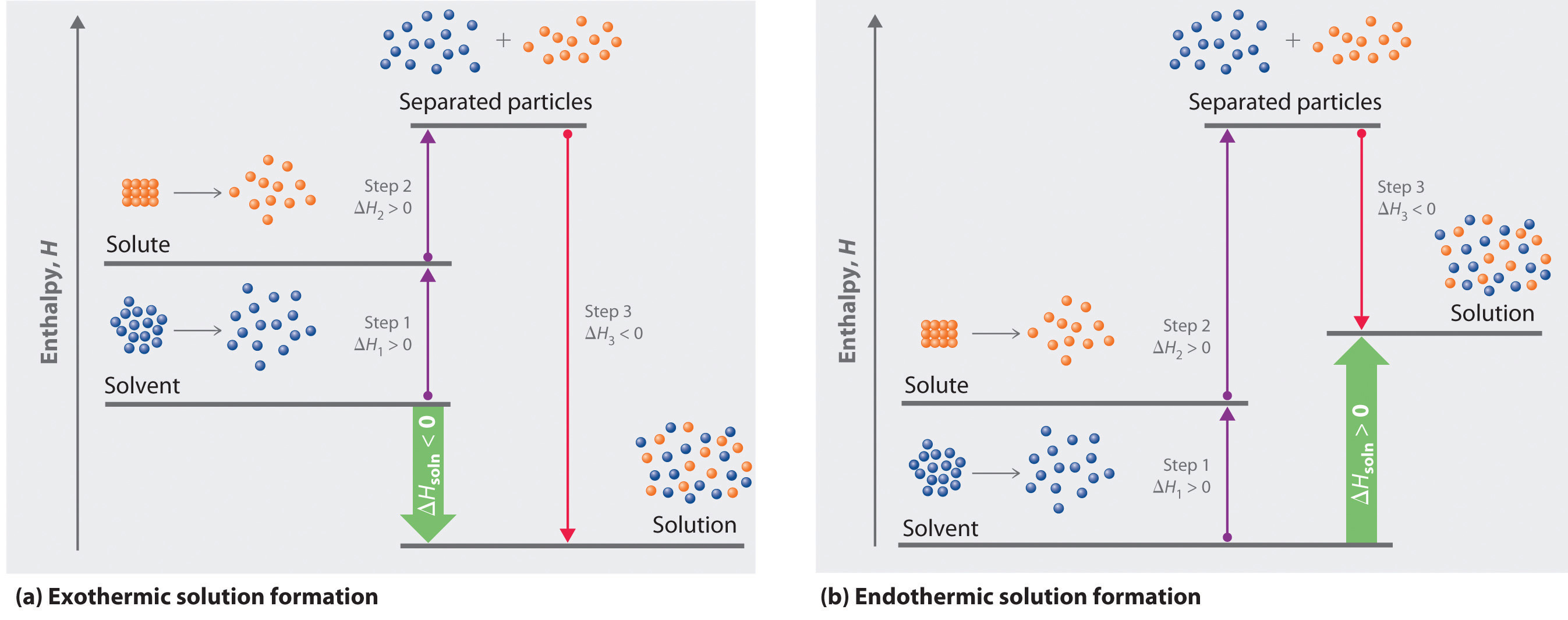
Breaking up the Solvent. Unreacted monomers and solvent stream 7 then continue onto a purge point stream 8 which represents venting and other losses and is required to prevent accumulation. One must be a solution and the other a pure solvent.
Water is considered to be a polar solvent because it has a positive H atom and negative O atom poles. The second process is very similar to the first step. Acetone is an organic solvent.
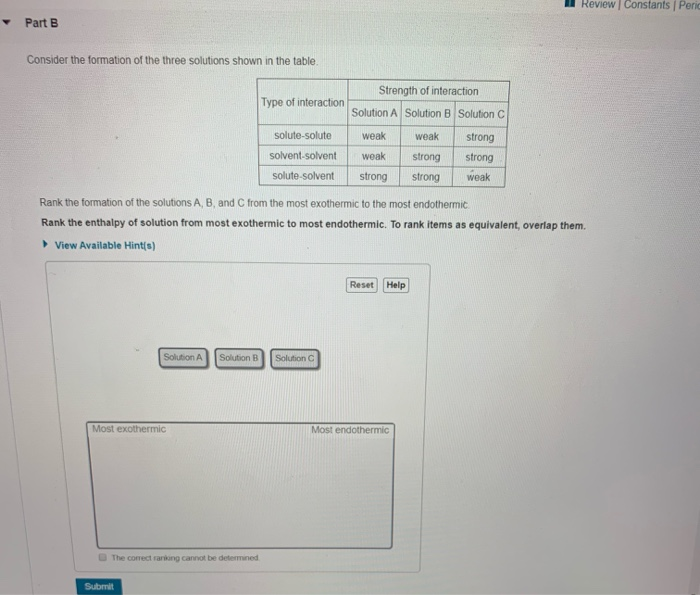
Van der Waals interactions are long range but are weak. 45 A metallic bond is the electrostatic attraction between a lattice of positive ions and delocalized electrons The strength of a metallic bond depends on the charge of the ions and the radius of the. Much like how the solute A needed to break apart from itself the solvent B also needs to overcome the intermolecular forces holding it together.
Energy released when a particle is physisorbed is of the same order of magnitude as the enthalpy of condensation. Hence the interactions between acetone molecules and polymer molecules of the nail polish are stronger than those between polymer molecules and the polymer turns from solid to liquid. The relative strengths of these interactions are London dispersion forces dipole-dipole forces hydrogen bonds Metallic bonding.
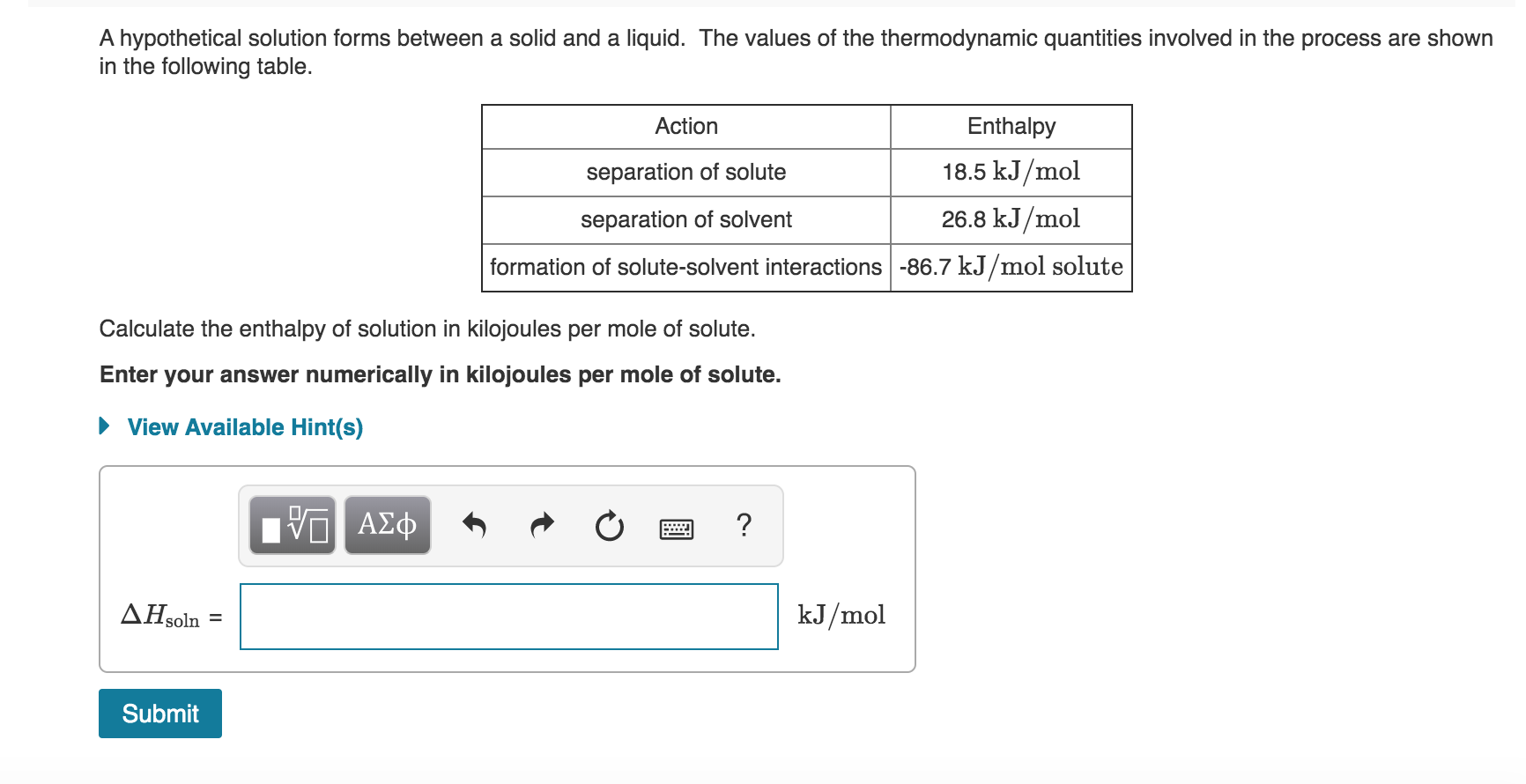
For a chemical reaction M g aq M aq Enthalpy change H Hyd. Concentrated electrolytes usually demonstrate good electrochemical performance and thermal stability and are also supposed to be promising when it comes to improving the safety of lithium-ion. The enthalpy of this process is called ΔH_2.
One of the liquids exhibits hydrogen bonding while the other has very strong dipole-dipole interactions. Polymer solvent unreacted monomers initiator and chain transfer agent flow out of the reactor to the separator stream 4 where polymer residual initiator and chain transfer agent are removed. This has to do with the polarity of each water molecule.
This causes the solvent molecules separate from each other. Their mixing must be an exothermic process. The hydrogen side of each water molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge.
Water is called the universal solvent because more substances dissolve in water than in any other chemical. Apparently the solvent resistance membranes are still porous and dissolution of organic extractant although reduced still exists.

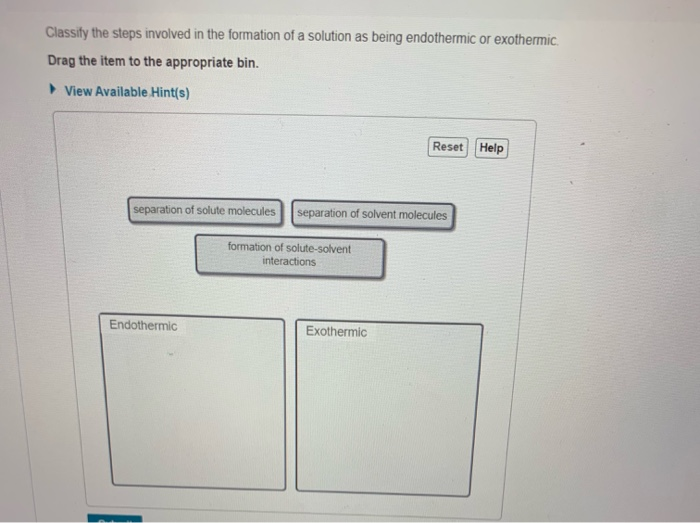
Solved 1 Energetics Of Solution Foration A Solution Is Fored When The Solute Uniformly Disperses Throughout Or Dissolves In The Solvent The Process Can Be Described Though Three Steps 1 Separation Of Solvent Solvent
Solved A Solution Is Formed When The Solute Uniformly Chegg Com
Solved A Solution Is Formed When The Solute Uniformly Chegg Com
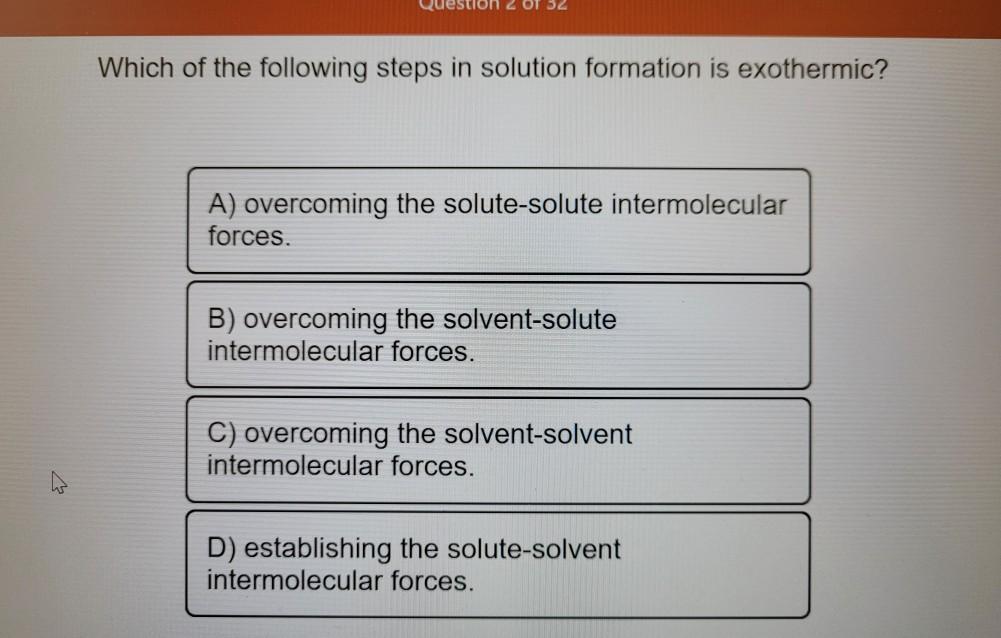
Solved Which Of The Following Steps In Solution Formation Is Chegg Com

Solved A Solution Is Formed When The Solute Uniformly Chegg Com

Chapter 13 Properties Of Solutions Created By Nick Brazones Brian Fike Maya Hairston Brian Kuttler Alex Reardon Chase Schilling Moses Suh Ppt Download
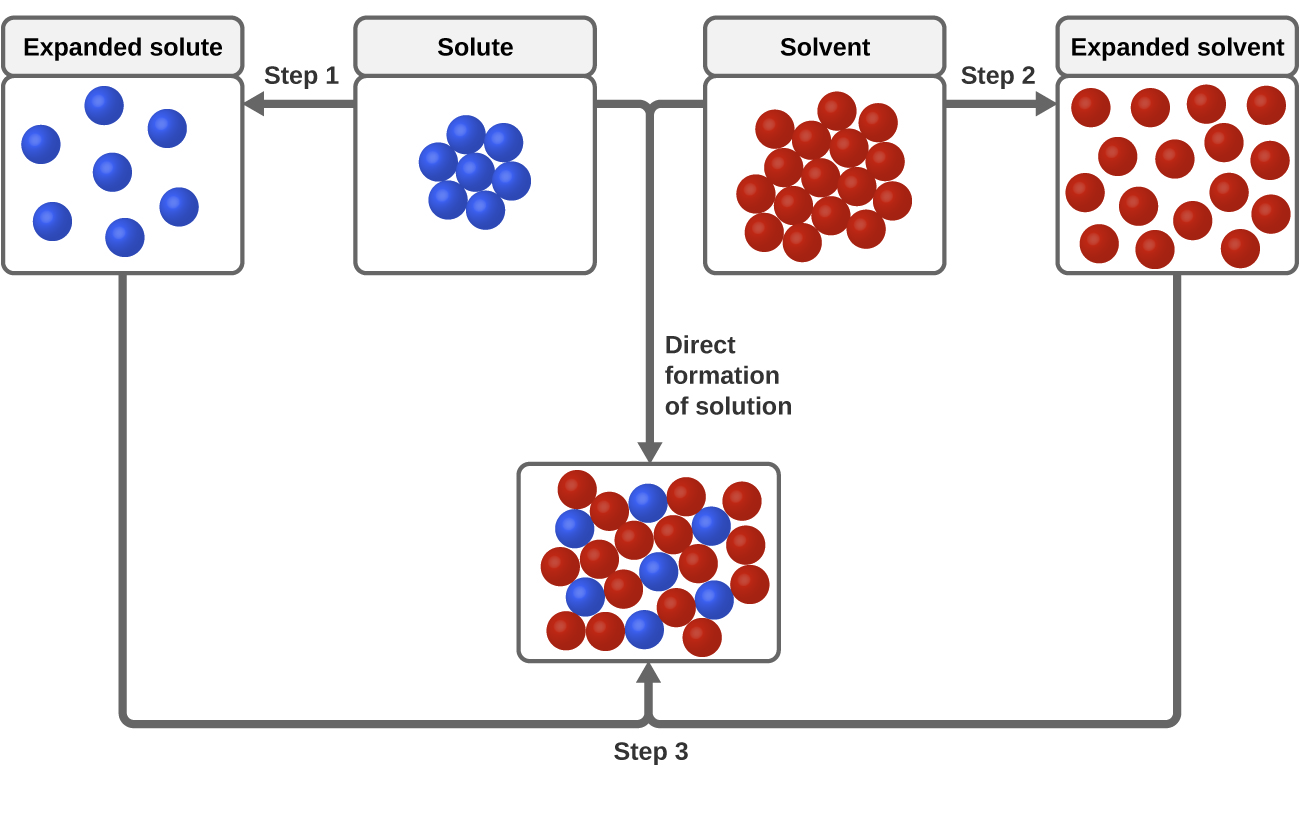
11 1 The Dissolution Process Chemistry

The Dissolving Process Is Exothermic When Youtube

Learn Solubility Solid In Liquid In 3 Minutes
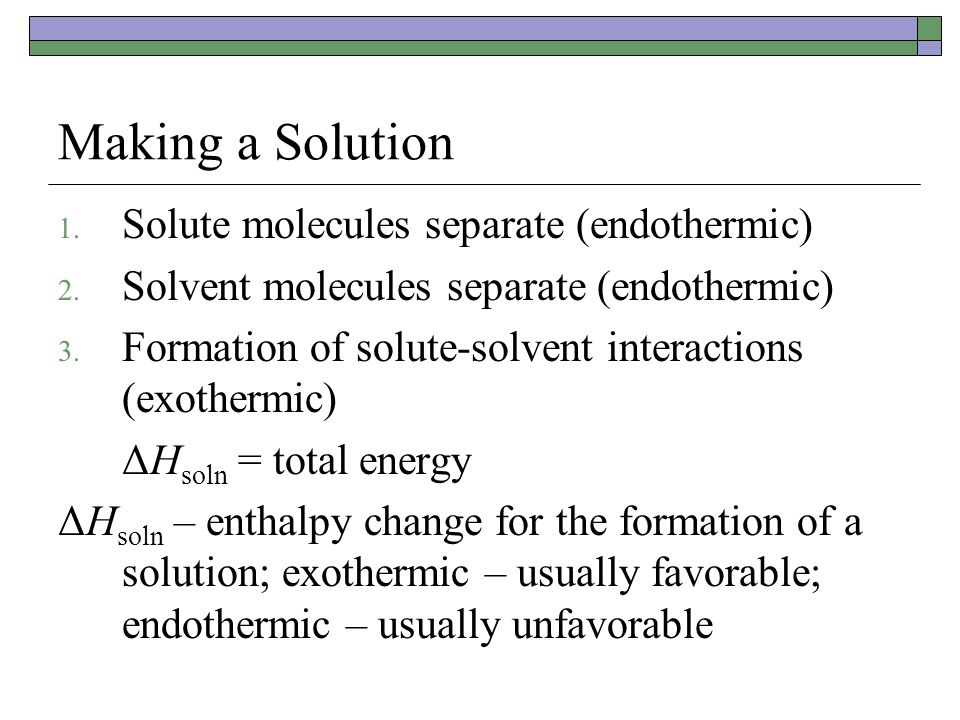
Properties Of Solutions Ppt Video Online Download

Chapter 13 Properties Of Solutions Ppt Download

13 3 Energetics Of Solution Formation Chemistry Libretexts

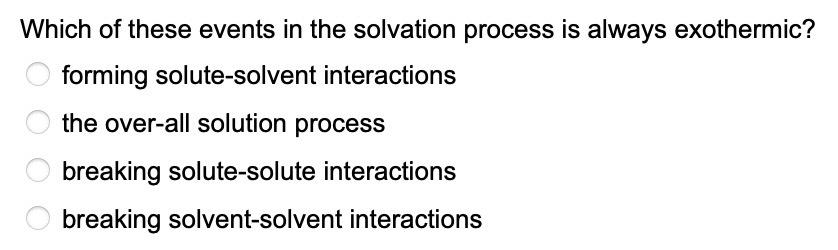
Solved Which Of These Events In The Solvation Process Is Chegg Com

Chapter 13 Properties Of Solutions Ppt Download

Solved If The Enthalpy Of Solution In Water Is Exothermic Which Of The Following Must Be True The Energy Required To Form Solvent Solute Interactions Is Small Adding Heat To The Solution Will Increase

Solved 5 5 In Making A Solution If Solute Solvent Chegg Com
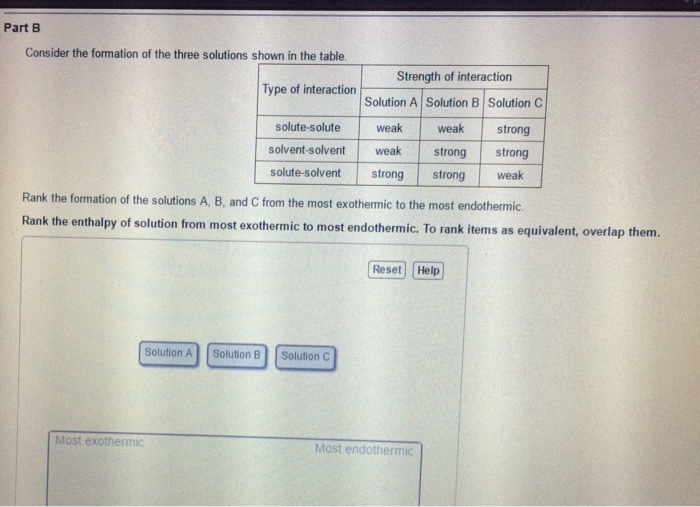
Solved T Energetics Of Solution Formation A Solution Is Chegg Com

Comments
Post a Comment